Heating Curve For Water Lab Answers . on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: finally, you will have the chance to revise your original answer. evaporation of sweat requires energy and thus take excess heat away from the body. an investigation can measure the temperature change through continuous heating of a substance. in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. the heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Working with a partner, sketch a heating curve for water at 101 kpa using the. Some of the water that you drink.
from www.chegg.com
on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: finally, you will have the chance to revise your original answer. the heating curve shown above is a plot of temperature vs time. Some of the water that you drink. Working with a partner, sketch a heating curve for water at 101 kpa using the. an investigation can measure the temperature change through continuous heating of a substance. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. evaporation of sweat requires energy and thus take excess heat away from the body. It represents the heating of substance x at a constant rate. in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective.
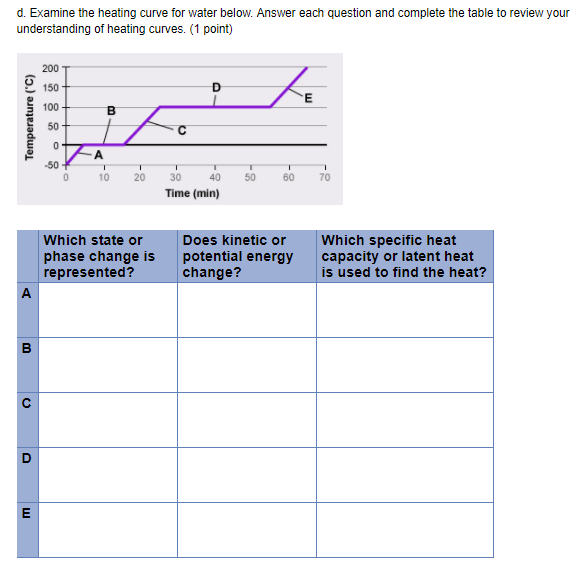
Solved d. Examine the heating curve for water below. Answer
Heating Curve For Water Lab Answers figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Working with a partner, sketch a heating curve for water at 101 kpa using the. finally, you will have the chance to revise your original answer. It represents the heating of substance x at a constant rate. in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: an investigation can measure the temperature change through continuous heating of a substance. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Some of the water that you drink. the heating curve shown above is a plot of temperature vs time. evaporation of sweat requires energy and thus take excess heat away from the body.
From printablelibagnames.z13.web.core.windows.net
Heating Curve Of Water Explained Heating Curve For Water Lab Answers in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. the heating curve shown above is a plot of temperature vs time. Working with a partner, sketch a heating curve for water at 101 kpa using the. finally, you will have the chance to revise your original answer. figure \(\pageindex{3}\). Heating Curve For Water Lab Answers.
From studylib.net
Lab Heating Curve Heating Curve For Water Lab Answers finally, you will have the chance to revise your original answer. on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Working with a partner, sketch a. Heating Curve For Water Lab Answers.
From studylib.net
Heating Curve of Water Lab (1) Heating Curve For Water Lab Answers an investigation can measure the temperature change through continuous heating of a substance. in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: the heating curve shown above is a. Heating Curve For Water Lab Answers.
From brainly.com
Examine the heating curve for water below. Answer each question Heating Curve For Water Lab Answers an investigation can measure the temperature change through continuous heating of a substance. It represents the heating of substance x at a constant rate. Some of the water that you drink. on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: Working with a partner, sketch a heating. Heating Curve For Water Lab Answers.
From www.chegg.com
Solved d. Examine the heating curve for water below. Answer Heating Curve For Water Lab Answers Some of the water that you drink. on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: finally, you will have the chance to revise your original answer. It represents the heating of substance x at a constant rate. Working with a partner, sketch a heating curve for. Heating Curve For Water Lab Answers.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Pdf Heating Curve For Water Lab Answers figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the heating curve shown above is a plot of temperature vs time. in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Some of the water that you drink. It represents. Heating Curve For Water Lab Answers.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Answers Heating Curve For Water Lab Answers finally, you will have the chance to revise your original answer. evaporation of sweat requires energy and thus take excess heat away from the body. Working with a partner, sketch a heating curve for water at 101 kpa using the. on the heating curve for water, the line that corresponds to vaporization is longer than the line. Heating Curve For Water Lab Answers.
From worksheetfullrusskies.z21.web.core.windows.net
Worksheet On Heating And Cooling Curves Heating Curve For Water Lab Answers on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. the heating curve shown above is a plot of temperature vs time. Working with a partner, sketch a heating curve for. Heating Curve For Water Lab Answers.
From www.scribd.com
Heating Curve of Water Worksheet Phase (Matter) Phase Transition Heating Curve For Water Lab Answers an investigation can measure the temperature change through continuous heating of a substance. Working with a partner, sketch a heating curve for water at 101 kpa using the. in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. the heating curve shown above is a plot of temperature vs time. Some. Heating Curve For Water Lab Answers.
From www.chegg.com
Solved Part B Heating Curve for Water Volume of water Heating Curve For Water Lab Answers in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. the heating curve shown above is a plot of temperature vs time. finally, you will have the chance to revise your original answer. It represents the heating of substance x at a constant rate. an investigation can measure the temperature. Heating Curve For Water Lab Answers.
From worksheetmagicrusso.z21.web.core.windows.net
Heating Curve Of Water Answers Heating Curve For Water Lab Answers evaporation of sweat requires energy and thus take excess heat away from the body. Working with a partner, sketch a heating curve for water at 101 kpa using the. the heating curve shown above is a plot of temperature vs time. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75. Heating Curve For Water Lab Answers.
From worksheetlistmo.z21.web.core.windows.net
Heating Curve Of Water Worksheets Answers Heating Curve For Water Lab Answers the heating curve shown above is a plot of temperature vs time. Working with a partner, sketch a heating curve for water at 101 kpa using the. It represents the heating of substance x at a constant rate. evaporation of sweat requires energy and thus take excess heat away from the body. figure \(\pageindex{3}\) shows a heating. Heating Curve For Water Lab Answers.
From studylib.net
Lab Heating Curve of Water Heating Curve For Water Lab Answers on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: Working with a partner, sketch a heating curve for water at 101 kpa using the. the heating curve shown above is a plot of temperature vs time. Some of the water that you drink. an investigation can. Heating Curve For Water Lab Answers.
From printablelibaccuses.z13.web.core.windows.net
Heating Curve Of Water Worksheets Heating Curve For Water Lab Answers evaporation of sweat requires energy and thus take excess heat away from the body. Some of the water that you drink. on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75. Heating Curve For Water Lab Answers.
From www.youtube.com
Heating Curve of Water Lab Time Lapse YouTube Heating Curve For Water Lab Answers evaporation of sweat requires energy and thus take excess heat away from the body. on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: It represents the heating of substance x at a constant rate. the heating curve shown above is a plot of temperature vs time.. Heating Curve For Water Lab Answers.
From studylib.net
Heating Curve Lab Heating Curve For Water Lab Answers on the heating curve for water, the line that corresponds to vaporization is longer than the line for melting, because: evaporation of sweat requires energy and thus take excess heat away from the body. in this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Some of the water that you drink.. Heating Curve For Water Lab Answers.
From quizzmagickellen99.s3-website-us-east-1.amazonaws.com
Worksheet Heating Curve Of Water Answers Heating Curve For Water Lab Answers Working with a partner, sketch a heating curve for water at 101 kpa using the. evaporation of sweat requires energy and thus take excess heat away from the body. finally, you will have the chance to revise your original answer. the heating curve shown above is a plot of temperature vs time. Some of the water that. Heating Curve For Water Lab Answers.
From worksheetfullcasandra.z5.web.core.windows.net
Heating Curve Of Water Worksheets Answers Heating Curve For Water Lab Answers finally, you will have the chance to revise your original answer. the heating curve shown above is a plot of temperature vs time. an investigation can measure the temperature change through continuous heating of a substance. figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water.. Heating Curve For Water Lab Answers.